Heartwarming Info About How To Draw Ionic Compounds
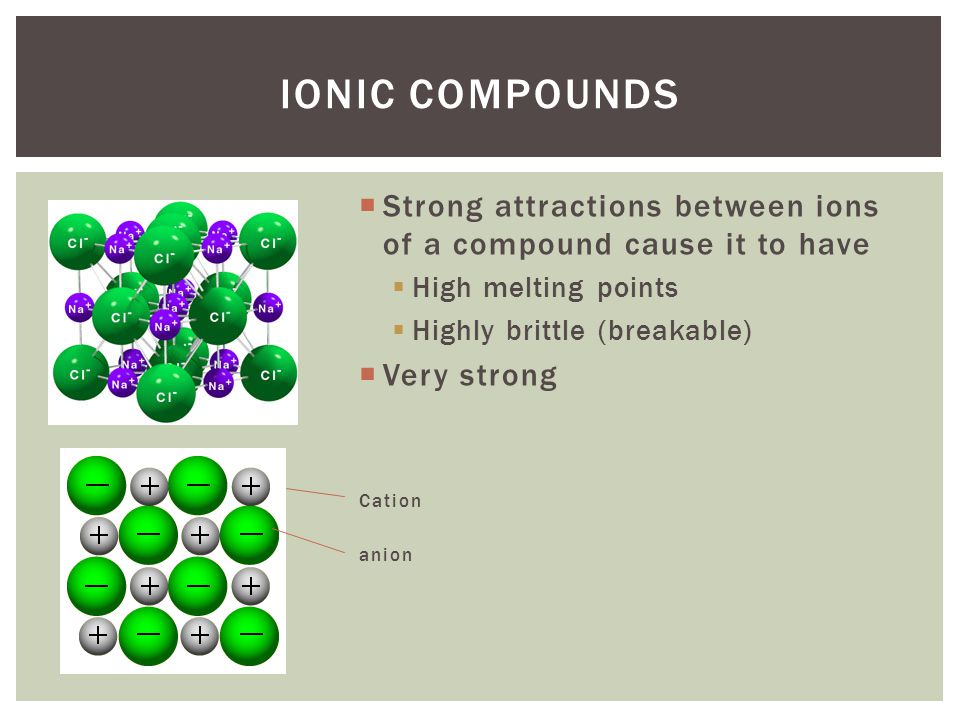
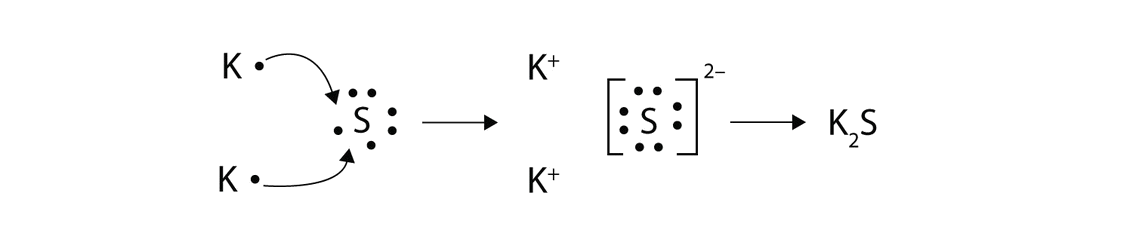
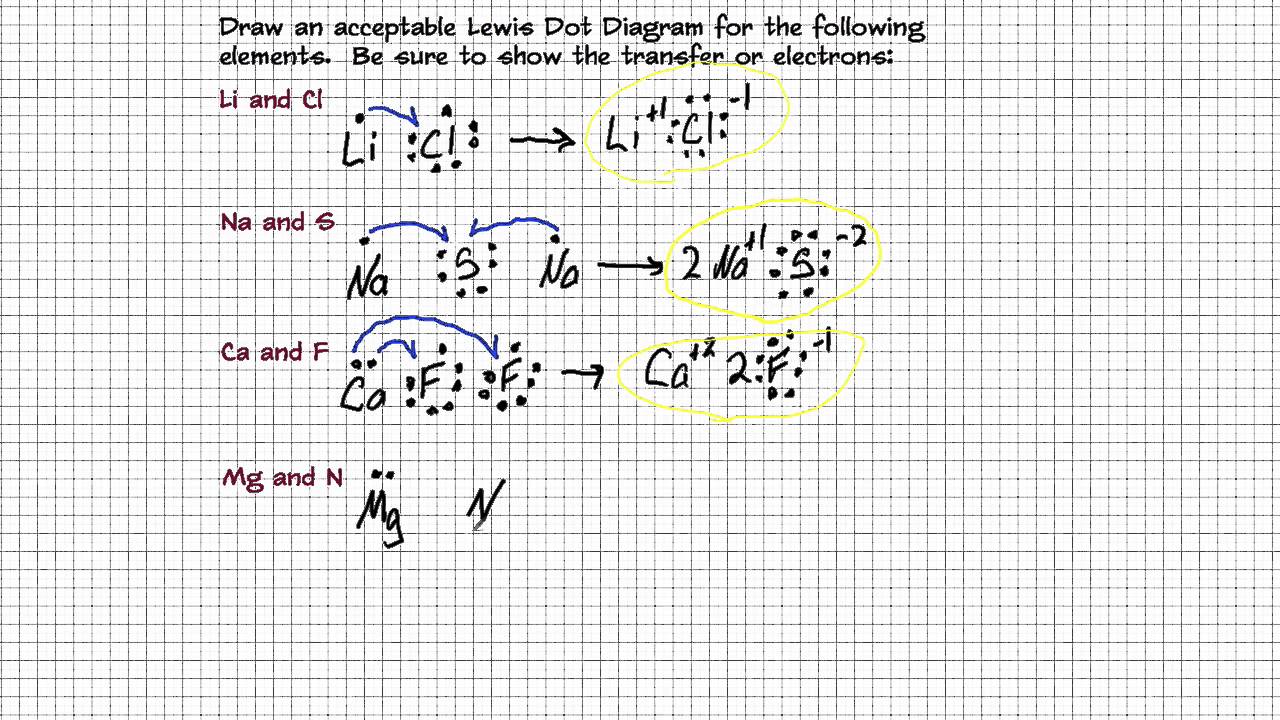
# of electrons lost= # of electrons gained 2.
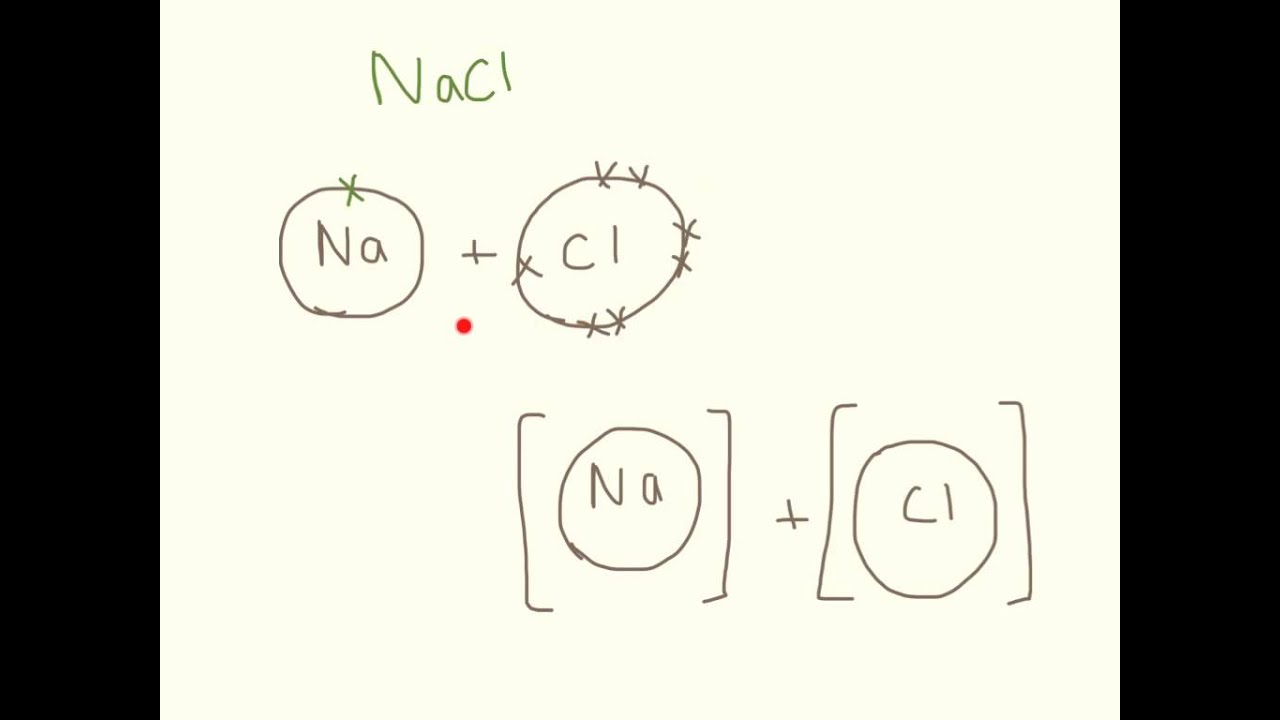
How to draw ionic compounds. The rules in naming ionic compounds are: Rewrite by separating the soluble ionic compounds into their dissociated ions. Draw a square bracket around each ion.
Examples include nacl, mgf2, k2o, and al2o3.my website: Always show the correct ratio of cations to anions to show a conservation of charge. When there are two or more of the same polyatomic ion in the formula.
Modeling ionic compounds with lewis dot structures write out the relevant ions with their charges and decide how many of each ion is required to make a balanced equation. The cation is written first in the name, followed by the anion. Shared pairs of electrons are drawn as lines between atoms,.
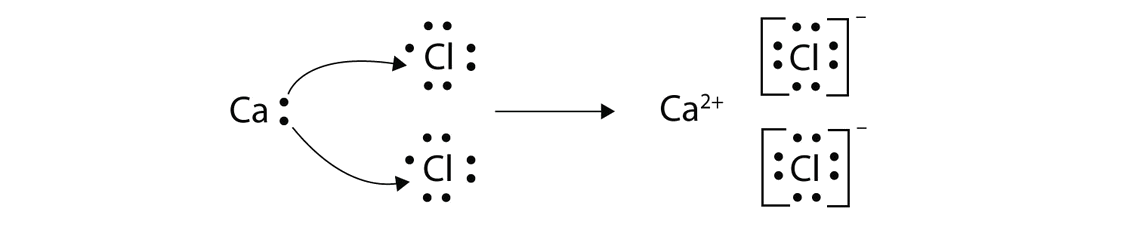
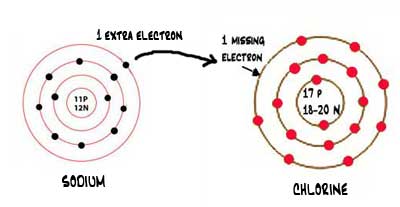
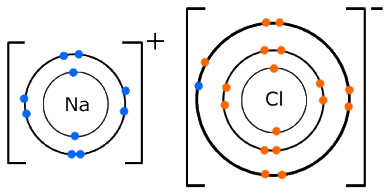
Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium chloride is formed. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Magnesium now has an empty third shell so draw the second shell instead.
For ionic compounds with polyatomic ions, like nano3 or k2so4, we need to draw the lewis structure for the polyatomic (which is usually a covalent compound where valence. Add the charge outside the brackets at the top right corner. An ionic compound is made up of charged particles, called ions.
They identify 8 compounds as. Drawing lewis diagrams for ionic compounds 1. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.




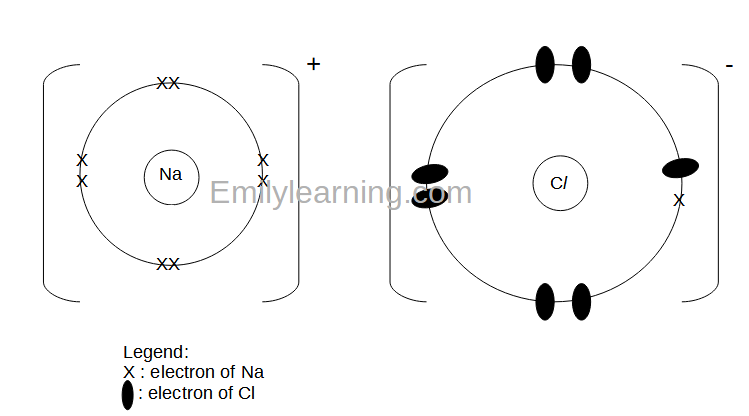

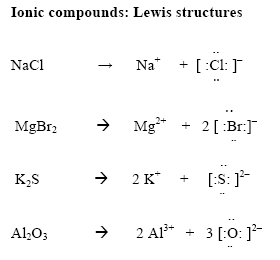



![How To Draw Dot And Cross Diagram Of Sodium Chloride Ionic Compound [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/10/thumb-nacl.jpg)
